In August Kite bought out Interius for $350M to enter the in-vivo CAR-T game. Now it made another bald move to step up its game.
Just last month, Kite Pharma’s announced a $1.64B collaboration ($120M upfront + up to $1.52 billion in milestone payments) with Pregene to co-develop the next-generation of in vivo CAR-T therapies.
But why exactly Pregene?
🧬 COMPANY’S FOCUS & TECHNOLOGY 🦠
Pregene is a chinese late clinical stage company that has developed proprietary engineering platforms for CAR-T, CAR-NK, TCR-T immunotherapies.
Pregene has some demonstrated technical assets that could help Kite strengthen its cell therapy portfolio:
- CAR Design and Discovery Platform: discovery platform focused on nanobodies that enables a high-throughput CAR design and optimization (for CAR-T, CAR-NK, TCR-T).
- Manufacturing: in-house manufacturing platforms for lentiviral production (serum-free, high-titer) combined with in-house capability across autologous and allogeneic modalities.
These manufacturing assets reduce reliance on external CDMOs and help speed development.
- Autologous and Allogeneic approaches: Pregene has developed both autologous and allogeneic clinical programs. Moreover it has already contributed to the development of EsoBiotec´s ESO-T01 in vivo CAR-T.
💊 CLINICAL PIPELINE 💊
Pregene’s programs span hematologic cancers and severe autoimmune disease:
AUTOLOGOUS CAR-T
Lead CAR-T program: PRG-1801, anti-BCMA
- Modality/Cargo: Autologous anti-BCMA CAR-T.
- Disease: Relapsed/refractory multiple myeloma.
- Clinical trials studying PRG-1801:
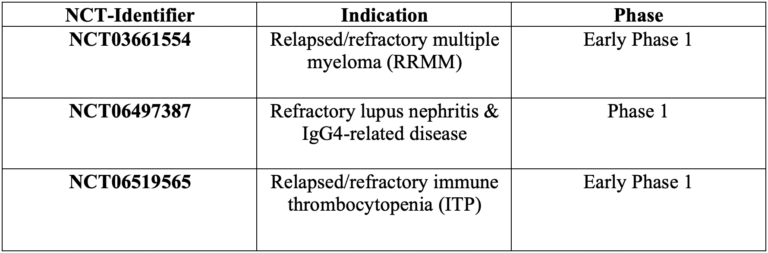
Pregene has closed different licensing deals involving PRG-1801:
– Greater China: licensing deal with Simcere Pharmaceutical
– EU and USA: licensing deal in 2022 with CellPoint (then acquired by Galapagos) for its development and commercialization (program later renamed GLPG-5301 and test in the PAPILIO-1 trial).
– India: licensing deal in 2021 with Dr. Reddy’s Laboratories
ALLOGENIC CAR-T
Allogeneic CAR-NK: PRG-2101 (anti-BCMA) – RRMM
- Modality: Off-the-shelf donor-derived CAR-NK targeting BCMA.
- Status: Phase 1 recruiting (China; NCT05652530)
IN VIVO CAR-T (with partners)
- EsoBiotech: Pregene contributed by providing their BCMA CAR transgene to EsoBiotec’s ESO-T01, the first-in-human in vivo anti-BCMA CAR-T.
- Kite-Pregene (2025): Collaboration to co-develop next-gen in vivo CAR-T across oncology/autoimmune.
HOW WILL PREGENE´S TECHNOLOGY HELP KITE IN VIVO THERAPY?
- Advanced Vector / Delivery Expertise: thanks to its high high-throughput platform for CAR-T combined with in-house manufacturing.
- Extended platform breadth: Pregene has developed expertise beyond CAR-T, including CAR-NK, dual-target CARs and TCR-T programs.
- Scalability: thanks to Pregene´s strong manufacturing capabilities
Pregene has already demonstrated expertise in in-vivo CAR-T, combined with strong high-throughputs CAR-T platforms and in house manufacturing of lentiviral vectors, making it a strong collaboration partner to advance Kite´s cell therapy programs.
LIMITATIONS
- Safety & Delivery: there are still a number of open questions around safety of in vivo CAR-T approaches, such as tropism, off-target effects, insertional oncogenesis and immune control. Unlike ex vivo CAR-T, in in vivo approaches QC of transduced cell before infusion is not possible.
- Lack of demonstrated clinical efficacy: to date, there aren´t robust clinical data to demonstrate neither the clinical efficacy nor the safety of in vivo CAR-T therapies.
- Manufacturing & Regulatory: scaling an in-vivo therapy – including vector production, biodistribution monitoring, and regulatory oversight of genome-engineering inside the patient – is still unproven at commercial scale.
- Competitive Landscape: many players are chasing in-vivo CAR-T therapies (e.g., LNP-based in-vivo CAR, non-viral approaches).
SUMMARY AND TAKE HOME MESSAGE
Following the acquisition of Interius, the addition of the collaboration with Pregene clearly aims at further strengthening Kite´s in vivo CAR T programs by adding new engineering modalities, manufacturing capabilities and expanded modality coverage.
So, essentially, Kite has pushed the gas pedal to strengthen every critical step of an in vivo CAR-T program (from viral delivery – CAR design – manufacturing) to bring it faster to the clinic, and then to market.
However, it remains crucial to wait for clinical proof-of-concept studies to assess potential limitations, and risks, of in vivo cell therapies.