As I briefly reported last week, in vivo CAR-T reprogramming programs are gaining momentum in the biotech space.
Following AbbVie’s acquisition of Capstan Therapeutics in June and AstraZeneca´s deal with EsoBiotec, last week Kite (a Gilead company) announced the complete buyout of Interius BioTherapeutics, a biotech company developing in vivo CAR-T and NK cell reprogramming platforms, for “only” $350 million USD in cash.
Let’s have a look at Interius’s scientific technology and clinical pipeline.
🧬 COMPANY’S FOCUS & TECHNOLOGY
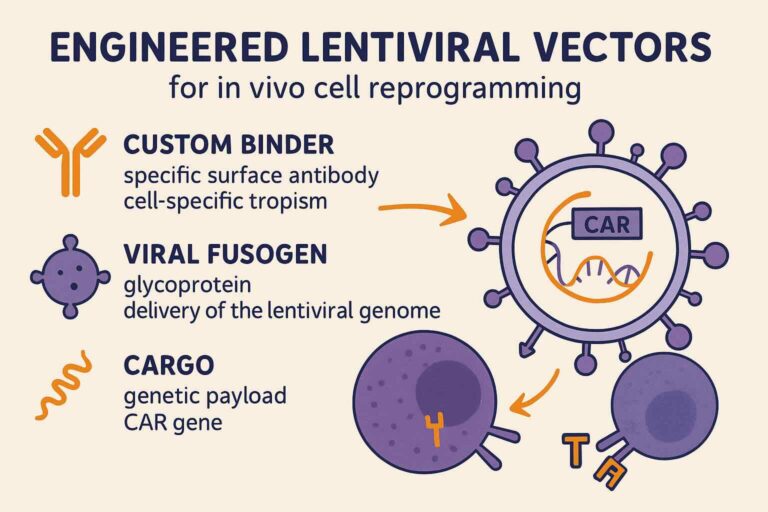
Interius has developed the next generation of engineered lentiviral vectors to precisely deliver genetic payloads to patient T and NK cells.
Interius’s lentiviral delivery system is based on 3 “magical ingredients” that make it a unique platform for in vivo cell reprogramming:
- CUSTOM BINDERS (specific surface antibodies): allow cell-specific tropism (i.e., targeting T and NK cells)
- VIRAL FUSOGEN: glycoprotein that allows the delivery of the lentiviral genome to the target cells
- CARGO (genetic payload inserted in viral genome): the CAR gene that will then be expressed in the target cells and reprogram them into CAR-expressing cells.
🧪 SCIENTIFIC APPLICATIONS
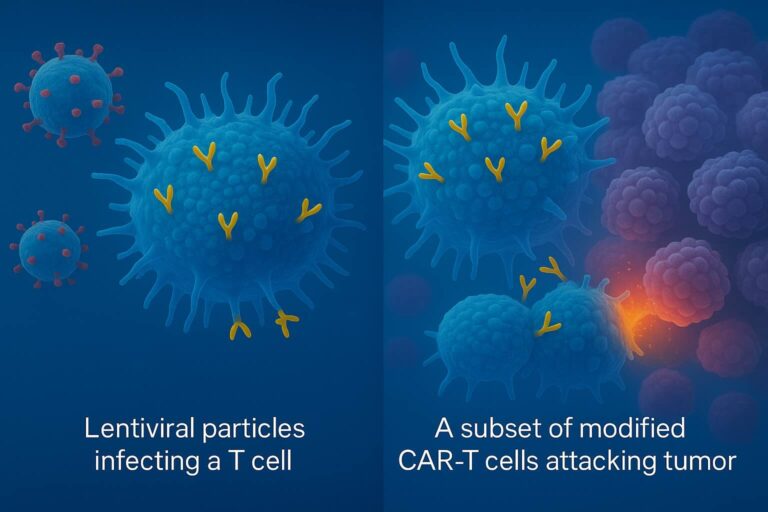
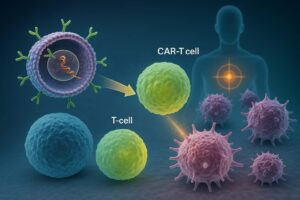
The main application of their platform has been in vivo reprogramming of CAR-T and NK cells.
How does Interius’s CAR-T reprogramming work:
- The lentiviral particles, thanks to an anti-CD7 surface antibody, bind to patient NK and T cells (CD7+) while CD7⁻ cells (i.e., B cells) are not infected, thus avoiding off-target infection.
- The viral fusogen mediates the entrance of the viral payload (CAR transgene) into the target cells (T and NK cells).
- T and NK cells start expressing the CAR gene and are effectively “reprogrammed” into CAR-T and CAR-NK cells, ready to kill malignant cells.
Interius’s viral vectors will deliver the CAR transgene directly to the patient’s T and NK cells in vivo, transforming them into CAR-T and CAR-NK cells that can target and kill cancer cells.
💊 CLINICAL PIPELINE
Interius’s pipeline program focuses on in vivo CAR-T reprogramming to treat B-cell malignancies and severe autoimmune diseases. Here are their top drug candidates (1):
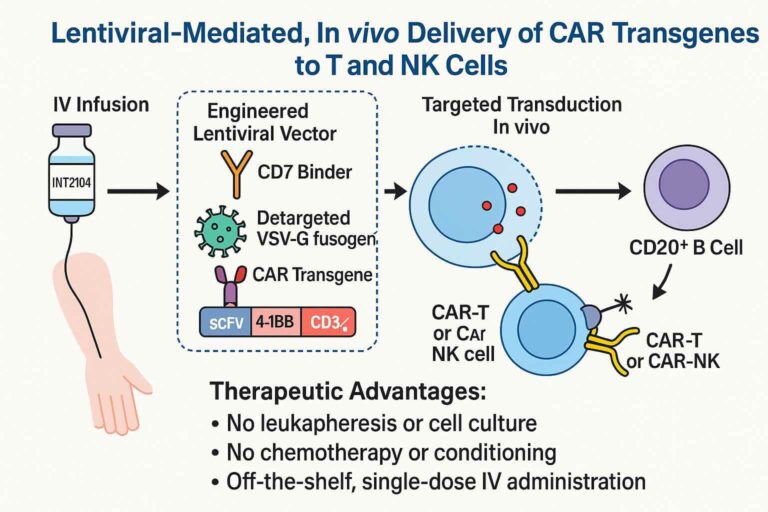
INT2104 (lead vector)
- Viral cargo: anti-CD20 CAR transgene
- Binder/Tropism: anti-CD7 antibody / T and NK cells
- Disease: B-cell malignancies
- Administration: single IV infusion
- Mode of action: in vivo reprogramming of patient T and NK cells to achieve elimination of malignant CD20+ B cells
- Clinical testing: recently entered a Phase I trial (INVISE) (2)
BONUS FACT: the INVISE trial is the first-in-human clinical trial testing an integrating in vivo CAR-T platform in Europe.
INT2106
- Viral cargo: anti-CD19 CAR transgene
- Binder/Tropism: anti-CD7 antibody / T and NK cells
- Disease: severe autoimmune diseases
- Administration: single IV infusion
- Mode of action: in vivo reprogramming of patient T and NK cells to achieve elimination of malignant CD19+ B cells
- Clinical testing: preclinical testing / IND-enabling
PRECLINICAL DATA OF INT2104
INT2104 showed very robust preclinical data. Let’s have a quick look at it (3):
IN VITRO: efficient transduction of human CD4+ and CD8+ T cells and NK cells, with no detectable transduction of B cells.
MOUSE MODELS: a single intravenous dose of INT2104 in humanized mice transplanted with human CD34+ cells resulted in the generation of CAR-positive T cells and subsequent complete elimination of B cells. Notably, treated mice also resisted tumor re-challenge, suggesting that the in vivo-generated CAR T/NK cells can provide lasting immune surveillance.
NON-HUMAN PRIMATE STUDIES: 15/16 monkeys showed a >75% reduction in circulating B cells. Importantly, neither off-target infections nor cytokine release syndrome (CRS) were detected, indicating a favorable preclinical safety profile. Toxicology studies showed no signs of toxicity with biodistribution specific to lymphoid tissues.
Overall, these preclinical data provided a very strong rationale for advancing INT2104 into first-in-human trials.
ADVANTAGES OF IN VIVO LENTIVIRAL CAR-T REPROGRAMMING
In vivo viral CAR-T reprogramming offers significant advantages compared to traditional ex vivo CAR-T preparation. Here are some of the most important ones:
- Off-the-shelf genetic medicine: personalized approach suitable for each patient
- Specific targeting: thanks to the custom binder (i.e., anti-CD7 antibody)
- One single IV infusion: has the potential to generate long-lasting immune surveillance (compared to the transient reprogramming of Capstan’s LNPs)
- No toxic lymphodepleting conditioning required
- Scalable manufacturing at lower costs
All these factors make in vivo viral CAR-T reprogramming an appealing platform that could overcome the traditional problems of high costs, complex manufacturing, and long waiting times associated with conventional ex vivo CAR-T generation.
However, it will be crucial to wait for the upcoming interim clinical data (expected by the end of 2025) to assess efficacy, safety profiles, and long-lasting effects.
OPEN QUESTIONS – FOOD FOR THOUGHTS
Although it goes beyond the scope of this post, in vivo CAR-T platforms still have some obstacles to overcome that could severely limit their application. For example:
- T-cell subsets infected: by using a CD7+ antibody to direct tropism, lentiviral vectors will transduce indiscriminately all T cells, including exhausted and senescent cells.
- Lack of multiplex engineering: so far, in vivo CAR-T platforms can only deliver one CAR (i.e., anti-CD19 CAR) and are thus far away from the 3rd/4th generation CARs generated ex vivo.
- Quality control (QC): while ex vivo CAR-T undergoes rigorous QC before infusion to the patient, in vivo CAR-T doesn’t allow precise QC assessment.
KEY TAKEAWAY MESSAGE
Interius’s in vivo CAR-T lentiviral platform has the potential to change the rules of the game in CAR-T therapy thanks to:
- Specific targeting (no apparent off-target effects)
- Off-the-shelf therapy: avoids high costs, manufacturing complexity, and is highly scalable
- Single infusion with long-lasting effects
However, there are still many open questions and technical aspects that need to be addressed in clinical studies.
Pingback: PREGENE x KITE: Kite double-downs on its in vivo CAR-T arsenal. – ianghezzi.com