Can natural cytotoxic molecules lead the way in anti-cancer therapies?
🧬 COMPANY’S FOCUS & TECHNOLOGY
Heidelberg Pharma is an oncology biotech company focused on the development of innovative antibody-drug conjugates (ADC) to treat different cancers.
ADCs allow the selective delivery and transport of cytotoxic molecules – or payloads – into cancer cells based on the specificity of the antibody binding; in this way, only cancer cells are targeted and killed, while sparing healthy cells (1).
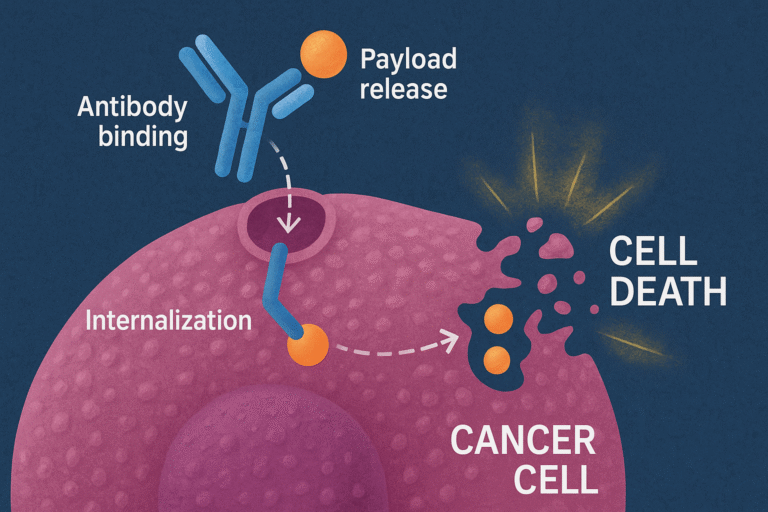
HeidelbergPharma has pioneered the development of ADC by using Amanitin as payload, a natural cytotoxic compound derived from green death cap mushroom, to kill cancer cells.
 HOW DOES ATAC (Amanitin-based ADC technology) WORK?
HOW DOES ATAC (Amanitin-based ADC technology) WORK?
The therapeutic success of Amanitin-based ADC technology (also called ATAC technology) is associated with its peculiar mechanism of action on cancer cells:
- Cell cycle independent killing of cancer cells: Amanitin can target dormant cancer stem cells, which usually escape conventional chemotherapeutics.
- Overcoming drug resistance: Amanitin is not a common substrate for drug efflux pumps, which are often exploited by cancer cells to eliminate therapeutics.
Amanitin binds to RNA polymerase II and thereby inhibits the cellular transcription process, causing the death of cancer cells.
💊 CLINICAL PIPELINE
The company has been expanding its ATAC-based portfolio across different cancer entities. The most advanced candidates are (1):

– ATAC targeting BCMA (B Cell Maturation Antigen; CD269) in Multiple Myeloma (MM).
– Currently being evaluated in a Phase I/IIa in progressive relapsed or refractory Multiple Myeloma.
– Promising results, in terms of safety and efficacy, have prompted a continuation of the trial in order to identify optimal dosing for a phase II trial (2).

– ATAC targeting CD37 in Non-Hodgkin lymphoma (NHL).
– At the preclinical level, HDP-102 showed significant anti-tumour activity and safety (3).
– Based on the promising preclinical data, a Phase I clinical trial of HDP-102 in NHL has been initiated and the first patient was recently dosed.