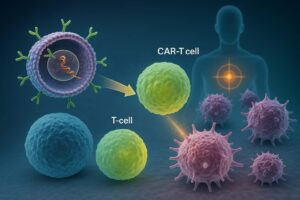
Wugen is a US-born and based biotech that develops off-the-shelf allogeneic CAR-T therapies to treat hematological malignancies.
In particular, Wugen has taken on the very difficult challenge of treating T-cell malignancies, which are notoriously complicated to target with immunotherapy due to two main challenges (1):
- Fratricide or friendly fire (CAR-T vs CAR-T): CAR-T cells themselves express the target antigen (CD7), which is also found on T-cell tumor cells. This can lead to CAR-T cells killing each other — hence the term “friendly fire” or “killing the good guys.”
- Graft vs. Host Disease (GvHD): the T-cell receptor (TCR) of allogeneic donor cells can potentially trigger GvHD in the patient.
Let’s look at how Wugen’s unique CAR-T platform addresses these challenges:
🧬 COMPANY’S FOCUS & TECHNOLOGY 🦠
Wugen’s allogeneic CAR-T platform builds on smart molecular designs:
- Collection of T cells from healthy donors
- Introduction of an anti-CD7 CAR into healthy donor cells
- CRISPR/Cas9 editing of these CAR-T cells to:
- Delete the CD7 gene: prevents CAR-T cells from expressing CD7 and killing each other (avoids fratricide).
- Knock out the TCR: eliminates the T-cell receptor, thereby eliminating the risk of GvHD.
In this way, Wugen’s CAR-T products effectively target only tumor T-cells while sparing CAR-T cells themselves and preventing immune rejection.
💊 LEAD CANDIDATE: WU-CART-007 💊
So far, Wugen has publicly shared one lead program, WU-CART-007, under development for a variety of indications. The most advanced program targets T-cell acute lymphoblastic leukemia (T-ALL) and lymphoblastic lymphoma (T-LBL).
WU-CART-007
- Cargo: 2nd generation anti-CD7 CAR transgene
- Tropism: anti-CD7 antibody / T-cells
- Disease: T-ALL/T-LBL
- Mode of action: CAR-T cells eliminate tumor T-cells while avoiding elimination of other T cells and preventing GvHD
- Clinical testing: Very promising Phase 1/2 results, showing remarkable anti-tumor activity with manageable toxicity (2)
At the same time, Wugen is testing WU-CART-007 in preclinical models of AML, and is carrying out a discovery phase in autoimmune diseases.
⚖️ CLINICAL REGULATORY APPROVAL
Key milestones for WU-CART-007 include:
- 2021: IND clearance and Orphan Drug designation from the FDA
- 2022: FDA grants Fast Track and Rare Pediatric Disease designations
- 2024: WU-CART-007 receives FDA Regenerative Medicine Advanced Therapy (RMAT) designation and EMA PRIority MEdicines (PRIME) status
These expedited designations highlight regulatory recognition of WU-CART-007’s potential to address unmet needs in pediatric T-cell cancers, which are marked by high relapse rates and mortality.
🧪 CLINICAL TRIAL TIMELINE
First-in-Human (Phase 1/2) Trial (NCT04984356) (2021–2023)
- Enrollment began in late 2021; by mid-2023, the recommended Phase 2 dose (RP2D) was determined at 900 million CAR-T cells.
- Patients: 28 adolescents and adults with highly aggressive T-ALL/LBL, most refractory to several prior treatments.
- Results at RP2D:
- Efficacy: Overall response (OR) rate of 91%, with complete remission (CR) in 72% of patients.
- Safety: Adverse events were manageable. Most patients developed cytokine release syndrome (CRS) after infusion, but cases were pharmacologically controlled.
Pivotal Phase 2 (T-RRex) Trial (NCT06514794) (2025–ongoing)
- Enrollment began in early 2025 at a global scale, including centers in the U.S., Europe, and Asia.
- Design: Global Phase 2, single-arm study in both pediatric and adult patients with R/R T-ALL/LBL.
- Primary endpoint: Efficacy and safety at RP2D.
- Cohorts:
- Patients with active relapsed/refractory disease
- Exploratory cohort of patients in minimal residual disease (MRD)-positive status
This positions Wugen’s WU-CART-007 as the first allogeneic CAR-T therapy for T-cell malignancies.
💰 FUNDING & INVESTMENT ROUNDS
Wugen has consistently attracted strong investor backing, enabling it to privately fund clinical trials of its lead compound.
- Series A (2020): $36–37 million, supporting the transition of WU-CART-007 from preclinical to clinical stage.
- Series B (2021): $172 million, accelerating the clinical trial and manufacturing scale-up.
- Series C (2025): $115 million, funding the T-RRex trial, regulatory filings in the U.S. and EU, and preparing for commercial-scale manufacturing.

💡 KEY TAKEAWAY MESSAGE 💡
Wugen’s WU-CART-007 has the potential to become the first approved allogeneic CAR-T therapy for T-cell malignancies, offering new hope to thousands of patients with previously dismal prognoses.
The company’s technology and clinical progress have also inspired strong investor trust, further reinforcing confidence in Wugen’s clinical program.