Last June, Abbvie confirmed the acquisition of Capstan Therapeutics for 2.1bn USD cash. Given the recent amount and volume of biotech M&As, this might not be surprising.
However, the interesting aspect of this deal is the drug pipeline of Capstan Therapeutics: at the moment, none of their therapies is currently in late clinical testing; indeed, their most advanced candidate has only recently entered a Phase I trial.
This clearly highlight the confidence of Abbvie in Capstan´s early phase pipeline that motivated such a considerable investment.
Let’s have a look at what makes Capstan stand out.
SCIENTIFIC CORE TECHNOLOGY
Capstan´s proprietary “CellSeeker™” platform is a targeted lipid nanoparticle (tLNP) system that allows the delivery of nucleic acids to specific cell types (i.e. CD8 T cells).
Briefly, tLNP are composed of novel lipid particles that are surface-conjugated with antibodies, thus allowing a highly specific targeting of a desired cell type (based on the surface antibodies used).
This technology has 3 key advantages:
- TARGETED DELIVERY: LNPs specifically target the desired cell type while not being up-taken by the liver.
- PAYLOAD FLEXIBILITY: LNPs can be loaded with different nuclei acids, for example: mRNA, siRNA and gene editors.
- IN VIVO CELL REPROGRAMMING: by delivering the paylod directly to the patients, specific cell types can be temporarily reprogrammed in vivo to instruct them with specific functions.
SCIENTIFIC APPLICATIONS: in vivo CAR-T reprogramming
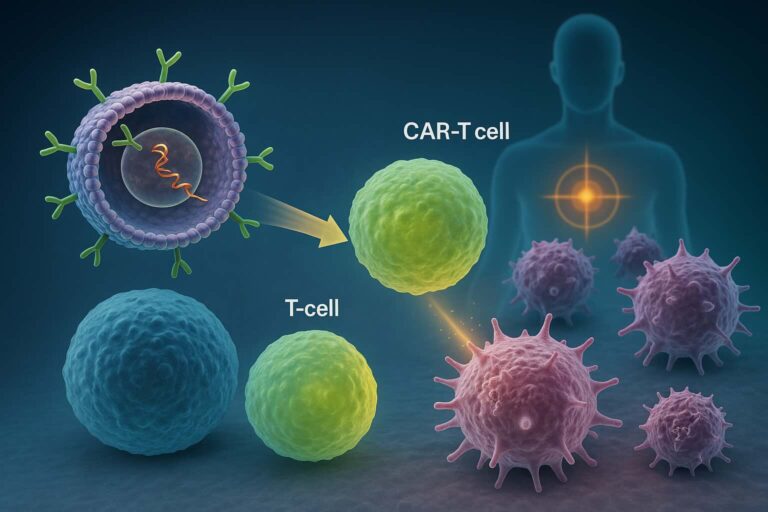
Capstan has predominantly exploited its technology to tackle an ambitious challenge: reprogramming CAR-T cells in vivo.
Here’s a breakdown of their workflow (1).
- LNPs are loaded with an anti-CD19 CAR mRNA and infused to the patient.
- LNPs, thanks to the anti-CD8 antibody on their surface, can specifically bind the patient CD8 T cells
- The anti-CD19 CAR mRNA is delivered and temporarily reprograms CD8 T cells in vivo
- Reprogrammed CD8 T cells will eliminate B cells, resulting in a transient depletion of malignant B-cell in blood and tissues.
This transient CAR-T reprogramming offers the conventional immunotherapy efficacy, with the additional benefits of avoiding prolonged persistence of CAR-T in the patients, and consequently long-lasting side effects.
CLINICAL PIPELINE
Capstan´s pipeline program focuses on in vivo CAR-T reprogramming in oncology, autoimmunity and fibrosis. Here are their top drug candidates:
CPTX2309 (lead program)
Target: CD19
Disease: autoimmune diseases
Mode of action: achieve a transient elimination of autoreactive B cells, allowing a subsequent restoration of healthy B cells (“immune reset”)
Clinical testing: recently entered a Phase I trial in healthy volunteers (2)
CPTX2506
Target: BCMA on plasma cells
Disease: oncology (multiple myeloma) and autoimmune diseases
Clinical testing: Discovery / Preclinical testing
FAP CAR-T (Fibroblast-targeting CAR-T)
Target: FAP (Fibroblast Activation Protein) on fibroblasts
Disease: fibrosis
Mode of action: elimination of pathogenic fibroblasts driving fibrosis
Clinical testing: Discovery / Preclinical testing
COMPETITORS IN in vivo CAR-T REPROGRAMMING
The two main competitor actively testing in vivo CAR-T reprogramming are:
- Interius BioTherapeutics with INT2104
- Umoja Biopharma with UB-VV111.
Despite both INT2104 and UB-VV111 having been already dosed in Phase 1 trials, they both rely on in vivo lentiviral-mediated reprogramming of patient T cells.
ADVANTAGES OF mRNA in vivo REPROGRAMMING
Compared to conventional ex vivo or in vivo CAR-T therapies, Capstain´s CellSeeker platform has important advantages:
- TRANSIENT mRNA EXPRESSION AND REPOGRAMMING: potentially safer while achieving durable results
- SCALABLE TECHNOLOGY: it does not require the usual complex manufacturing processes used for ex vivo therapies
- TOLERABILITY: patients do not need to undergo heavy lymphodepleting conditioning
- OFF THE SHELF: patient own cells are directly repgroammed in vivo.

Pingback: Kite (a Gilead company) joins the in vivo CAR-T race – ianghezzi.com